Download this article in PDF format.
What you’ll learn:
- Remote wireless applications tied to the industrial Internet of Things (IIoT) demand ultra-long-life, industrial-grade lithium batteries.
- To conserve energy, low-power devices rely on industrial-grade primary (non-rechargeable) lithium batteries when average current is in microamps.
- If average current is in milliamps, then it may require the use of an energy-harvesting device along with an industrial-grade Li-ion rechargeable battery.
- Extended battery life demands very low annual self-discharge by harnessing the passivation effect.
- Harsh operating environments reduce battery performance and accelerate battery self-discharge.
- Wireless applications that utilize two-way wireless communications require a hybrid solution.
Indoor wireless applications have much different power requirements than remote outdoor locations. Indoor applications are generally more accessible for easy battery replacement, and moderate temperatures serve to maximize battery life, commonly enabling the use of inexpensive consumer-grade alkaline and lithium-ion (Li-ion) rechargeable batteries.
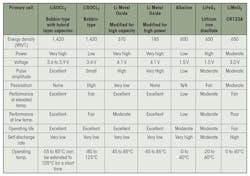
The opposite is true for remote wireless applications, particularly those connected to the industrial Internet of Things (IIoT) that experience prolonged exposure to extreme temperatures. These applications demand the use of robust, long-life, industrial-grade lithium batteries that can achieve extended battery life to reduce the total cost of ownership.
Some harsh environments are typical to remote locations, such as sweltering deserts, the Arctics, and pressurized undersea environments. However, extreme temperatures can also occur in more commonplace applications like automotive windshields, where the battery must perform reliably despite the severe temperature cycles that characterize car interiors.
That application requires the use of bobbin-type lithium-thionyl-chloride (LiSOCl2) cells, uniquely designed to handle heat soak, which can rise to 113°C (according to SAE) when parked, then rapidly cool to room temperature (see table). In cold weather, the opposite occurs: the battery must endure cold soak followed by a rapid rise in temperature.
Comparison of popular primary cells.
This example illustrates how prolonged exposure to extreme temperatures can negatively impact ordinary batteries due to frozen chemicals, voltage drops, and accelerated self-discharge: environmental challenges that limit the choice of battery.
Two Types of Low-Power Wireless Applications
Low-power remote wireless sensors and devices are increasingly utilized in applications such as asset and animal tracking, system control and data automation (SCADA), AMR/AMI utility metering, environmental and seismic monitoring, M2M, AI, and machine learning, to name a few.
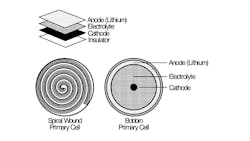
The typical low-power device that draws average current measurable in microamps may be suitable for various primary (non-rechargeable) batteries, including iron disulfate (LiFeS2), lithium manganese dioxide (LiMNO2), LiSOCl2, alkaline, and lithium metal oxide (Fig. 1).
1. Cells are typically implemented as a spiral-wound or bobbin-oriented configuration.
If the device draws average current measurable in milliamps, enough to prematurely exhaust a primary lithium battery, then it may call for some form of energy-harvesting device coupled with an industrial-grade, rechargeable Li-ion cell. Photovoltaic (PV) panels are the most common type of energy-harvesting device. However, certain niche applications also draw small amounts of current from equipment movement, vibration, temperature variances, and ambient RF/EM signals. Real-life examples are highlighted later in this article.
Every Remote Wireless Application is Unique
There are no one-size-fits-all solutions for battery-powered devices, as numerous factors affect the choice of battery chemistry. These variables include the amount of current consumed during active mode (including the size, duration, and frequency of pulses); energy consumed during “standby” mode (the base current); storage time (as normal self-discharge during storage diminishes capacity); thermal environments (including storage and in-field operation); and equipment cutoff voltage, which drops as cell capacity is exhausted or during prolonged exposure to extreme temperatures. Perhaps most critical is the annual self-discharge rate of the cell, which often exceeds the actual amount of energy consumed annually by the device.
As the lightest non-gaseous metal, with a high intrinsic negative potential that exceeds all others, lithium offers unique performance characteristics. They include the highest specific energy (energy per unit weight) and energy density (energy per unit volume) of all commercially available chemistries. Lithium cells operate within a normal operating current voltage (OCV) ranging from 2.7 to 3.6 V. Lithium chemistries are also non-aqueous, thus less likely to freeze in extreme cold.
If an ultra-long-life battery is required, the preferred choice is LiSOCl2 chemistry. These batteries can be constructed in two ways—bobbin-type or spirally wound.
Spiral-wound construction delivers greater surface area contact between layers, resulting in higher energy flow potential with higher self-discharge. Conversely, bobbin-type battery construction offers higher capacity, higher energy density, plus a wider temperature range (−80 to 125°C), along with a glass-to-metal hermetic seal to help prevent leakage. With reduced surface area for chemical reactions to occur, bobbin-type LiSOCl2 batteries can deliver an incredibly low self-discharge rate (under 1% per year for certain cells), enabling up to 40-year battery life.
Understanding the Passivation Effect
Battery self-discharge is ubiquitous, affecting all cells, including those that are disconnected. This naturally occurring phenomenon is mainly influenced by the passivation effect.
Passivation develops when a thin film of lithium chloride (LiCl) forms on the surface of the lithium anode, creating a temporary separation between the anode and the cathode, reducing the chemical reactions that cause self-discharge. Whenever a current load is placed on the cell, the passivation layer offers initial high resistance, causing a temporary drop in voltage until the discharge reaction begins to dissipate the passivation layer. This process keeps repeating every time the load is removed.
The level of passivation can be variable based on a cell’s current discharge capacity, the length of storage, storage temperature, discharge temperature, and prior discharge conditions. Partially discharging a cell and then removing the load tends to increase the amount of passivation relative to when the cell was new.
While passivation is extremely useful for minimizing battery self-discharge, too much of it can be problematic by blocking energy flow. The test of a battery manufacturer is to effectively control passivation to strike the ideal balance between energy flow and self-discharge.
A battery’s self-discharge rate is also influenced by the cell’s current discharge potential, its method of manufacturing, and the quality of its raw materials. As a result, a top-quality bobbin-type LiSOCl2 cell can achieve a self-discharge rate of 0.7% per year, thus retaining 70% of its original capacity after 40 years. By contrast, an inferior quality bobbin-type LiSOCl2 cell can have a self-discharge rate of up to 3% per year, thus losing as much as 30% of its capacity every 10 years, making 40-year battery life impossible.
Design engineers need to be fully aware that a battery’s actual self-discharge may take years to become fully measurable, and theoretical test data tends to be far less reliable than actual in-field data. So thorough due diligence is required when evaluating competing battery suppliers.
The Increasing Need for High Pulses in Two-Way Wireless Connectivity
More and more, remote wireless devices connected to the IIoT require periodic high pulses to power two-way wireless communications. To satisfy this added power demand, smart devices must incorporate a low-power communications protocol (i.e., WirelessHART, Zigbee, or LoRa) along with a low-power chipset and energy-saving components. Proprietary energy-saving techniques also are deployed to extend battery life.
Unfortunately, despite their incredible longevity, standard bobbin-type LiSOCl2 cells aren’t well-suited for delivering high pulses. However, that challenge can be easily overcome by adding a patented hybrid layer capacitor (HLC). The bobbin-type LiSOCl2 cell serves to deliver low-power background current while the HLC acts like a rechargeable battery to store pulses of up to 15 A. The patented HLC also features a unique end-of-life voltage plateau that can be interpreted and then programmed to deliver “low battery” status alerts.
Supercapacitors are commonly used to store high pulses for consumer electronics. However, they’re rarely used in industrial applications due to inherent limitations, including short-duration power; linear discharge qualities that don’t permit full discharge of available energy; low capacity; low energy density; and a very high self-discharge rate of up to 60% per year. In addition, supercapacitors linked in series require bulky cell-balancing circuits that add cost and draw more energy to further shorten their operating life.
Bobbin-Type LiSOCl2 Batteries in the Real World
Below are some representative examples of applications in harsh environments that often benefit from the use of long-life bobbin-type LiSOCl2 batteries:
Oceantronics
To solve the problem of transporting large, bulky scientific equipment across the Artic, Oceantronics redesigned the battery pack for its GPS/ice buoy. It replaced an oversized battery pack made up of 380 alkaline D cells with a far smaller, lighter, and more cost-efficient solution using 32 high-energy-density bobbin-type LiSOCl2 cells and 4 HLCs (Fig. 2).
2. Oceantronics redesigned the battery pack for its GPS/ice buoy, replacing 380 alkaline D cells with a far smaller, lighter, and economically designed battery pack using 32 bobbin-type LiSOCl2 cells and four HLCs. The redesigned battery pack reduced size and weight by 90% (54 kg down to 3.2 kg), making the device far easier to transport via helicopter to icebergs near the North Pole. (Courtesy of Oceantronics)
By achieving a 90%+ reduction in size and weight (54 kg down to 3.2 kg), the redesigned battery pack was far easier to transport to icebergs located near the North Pole via helicopter. In addition, converting from alkaline to bobbin-type LiSOCl2 chemistry multiplied potential battery life many times over.
Southwire
Minimizing size and weight is a major concern for utility line crews climbing up and down tall towers to install line/connector sensors that monitor the status of electric power transmission lines, including temperature, catenary, and line current. Southwire chose bobbin-type LiSOCl2 batteries to enable the collection and aggregation of data that’s transmitted via cellular network to warn a utility if a transmission line goes down.
Use of a bobbin-type LiSOCl2 battery enables the line/connector sensor to be more compact and lightweight (3.5 lbs.), providing easier portability in extreme temperatures (−40 to 50°C). These batteries also deliver the high energy density and high capacity required to provide 45+ days of backup power in case no line current is detected (Fig. 3).
3. Bobbin-type LiSOCl2 cells reduce the size and weight of Southwire’s line/connector sensors that monitor the temperature, catenary cables, and line current of electric power to warn the utility if power transmission lines go down. (Courtesy of Southwire)
Cryoegg
Beneath glaciers in Greenland and Antarctica lay water channels up to 2.5 km deep. Researchers are using wirelessly connected sensors to measure how climate change and rising sea levels are impacting these deep-water channels.
To support this research, the “Cryoegg” was developed by Cardiff University. This innovative device measures and transmits data regarding temperature, pressure, and electrical connectivity via underwater radio signals, eliminating the need for bulky and expensive cables that can become broken or disabled by glacial movement. Bobbin-type LiSOCl2 cells were chosen for their high capacity and energy density, extended temperature range, and ability to generate periodic high pulses to transmit data twice each day for approximately two years (Fig. 4).
4. Bobbin-type LiSOCl2 batteries feature higher capacity and energy density, an annual self-discharge rate as low as 0.7% per year to permit up to 40-year battery life, and the widest possible temperature range (−80 to 125°C).
The Cryoegg utilizes the same 169-MHz Wireless M-Bus radio technology that’s powered AMR/AMI utility meter transmitter units (MTUs) for nearly 40 years. Virtually all MTUs are powered by ultra-long-life, bobbin-type LiSOCL2 batteries that reduce the potential for costly system-wide battery failures, thereby reducing the potential for chaotic disruptions to normal billing systems as well as disabling remote start-up/shut-off capabilities.
Higher Energy Demand May Require Energy Harvesting
As previously noted, certain wireless applications that draw milliamps of current may demand the use of an energy-harvesting device in combination with Li-ion rechargeable batteries.
Consumer-grade rechargeable Li-ion cells are too short-lived for most industrial-grade applications, with a limited operating life of five years and 500 recharge cycles, restricted to use in moderate temperatures (0 to 40°C). Consumer Li-ion rechargeable batteries are also unable to deliver the high pulses required for two-way wireless communications.
By contrast, industrial-grade Li-ion batteries can operate for up to 20 years and 10,000 full recharge cycles at an expanded temperature range (−40 to 85°C), along with the ability to deliver periodic high pulses to power two-way wireless communications.
One prime example involves the use of small solar (PV) panels and industrial-grade Li-ion batteries to power collars that remotely monitor and communicate the health, location, and safety of animal herds. Similarly, solar/Li-ion hybrid systems are powering parking-meter fee-collection systems equipped with AI-enabled sensors to identify open parking spots.
Solar-powered devices require PV panels and rechargeable batteries large enough to power the device for five to seven days in case there’s no sunshine. While this scenario may rarely occur, the power supply must be overdesigned to cover any shortfall. Incorporating a primary LiSOCl2 cell can create a backup solution for charging the Li-ion cell to compensate for a prolonged period of no sunshine. This backup solution permits the use of a smaller PV panel and fewer or smaller Li-ion batteries, thus saving money while aiding in product miniaturization.
Rapid expansion of the IIoT is creating dynamic opportunities for industrial-grade, long-life primary and rechargeable Li-ion batteries that improve product reliability and reduce the total cost of ownership. If your application requires a battery that can last as long as your device, then do your due diligence and challenge potential battery suppliers to supply well-documented long-term test results, comprehensive in-field performance data (under similar conditions), and numerous customer references.
Sol Jacobs is VP and General Manager of Tadiran Batteries.