What you’ll learn:
- Why skin patches are a popular biosensor.
- How new fabrication approaches are enabling development of less obtrusive skin-path sensors.
- How advanced skin patches can monitor multiple biologic parameters.
Lots of biomedical-sensing activity is currently going on at universities and other research institutions, in many forms. While some of it involves sensors and circuitry that are implanted in a test subject, much of the research also centers on skin-mounted patches (sometimes called health patches) of various types.
This makes sense not only for the target patient, of course, but for the research team. After all, would you rather be implanting your device under test into the carcass of an animal (in vitro) such as a pig (very popular) or—even more challenging—into a live one (in vivo), or would you prefer dealing with a stick-on patch, with which you can easily get volunteers while minimizing regulatory and sanitary issues? Furthermore, patches avoid the many of the material and biocompatibility issues associated with implants.
To give you a sense of the very different skin-patch efforts underway, here’s a roundup of a few from different universities, with a range of objectives and implementations.
Cambridge University's Bioelectronics for Skin-Mounted Sensors
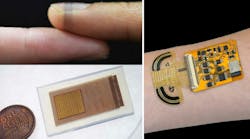
Researchers at Cambridge University (UK) have developed a way of making high-performance bioelectronics that can be customized to a wide range of biological surfaces, from a fingertip to the fluffy seedhead of a dandelion, by printing them directly onto that surface. Their technique takes its inspiration in part from spiders, which create sophisticated and strong web structures adapted to their environment, using minimal material.
The researchers spun their bioelectronic “spider silk” from PEDOT:PSS (a biocompatible, conducting polymer widely used in these sorts of projects), hyaluronic acid, and polyethylene oxide. The high-performance fibers were produced from a water-based solution at room temperature, which enabled the researchers to control the “spinnability” of the fibers. The researchers then designed an orbital spinning approach to allow the fibers to morph to living surfaces, even down to microstructures such as fingerprints.
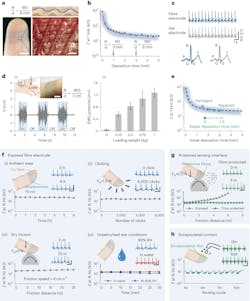
Despite its apparent fragility, the fiber-fabrication process doesn’t require a clean room, and the exposed fiber is mechanically stable and surprisingly rugged (Fig. 1).
The tests they ran with volunteers (humans, of course) included electrocardiogram and pulse assessments (Fig. 2).
The work is detailed in their paper “Sustainable and imperceptible augmentation of living structures with organic bioelectronic fibres” published in Nature.
UCSD's Wearable Ultrasound Patch for Continuous Brain Monitoring
Over at the University of California at San Diego, engineers developed a wearable ultrasound patch that can offer continuous, non-invasive monitoring of blood flow in the brain. The soft and stretchy patch can be comfortably worn on the temple to provide three-dimensional data on cerebral blood flow, which they claim is a first in wearable technology.
The current clinical standard, called Transcranial Doppler (TCD) ultrasound, requires a trained technician to hold an ultrasound probe against a patient’s head. The process is operator-dependent, so the accuracy of the measurement can vary based on the operator’s skill, and it’s impractical for long-term use. In contrast, the UCSD wearable ultrasound patch offers a hands-free, consistent, and comfortable solution that can be worn continuously during a patient’s hospital stay.
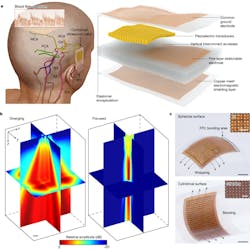
The patch, roughly the size of a postage stamp, is constructed from a silicone elastomer embedded with several layers of stretchy electronics. One layer consists of an array of small 2-MHz piezoelectric transducers, which produce ultrasound waves when electrically stimulated and receive ultrasound waves reflected from the brain.
Another key component is a copper mesh layer—made of spring-shaped wires—that enhances signal quality by minimizing interference from the wearer’s body and environment. The rest of the layers consist of stretchable electrodes (Fig. 3).
The 2-MHz ultrasound waves reduce the attenuation and phase aberration caused by the skull, and the copper-mesh shielding layer provides conformal contact to the skin while improving the signal-to-noise ratio (Fig. 4). Focused ultrasound waves support continuous recording of blood flow spectra at selected locations.
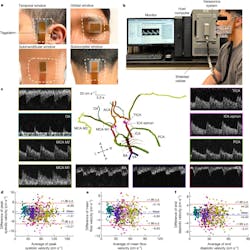
Their design requires advanced algorithms along with a significant amount of post-data-acquisition computation, but that’s an acceptable tradeoff (Fig. 5). Tests on 36 subjects showed close agreement with the medical-standard TCD instrumentation. Note that the figures of merit they use for quantifying accuracy are quite very different than that used for ultrasound technology for non-medical and even non-cranial assessments.
The paper “Transcranial volumetric imaging using a conformal ultrasound patch” published in Nature provides the details.
Caltech's Multimodal Sensor for Stress Assessment
Employing a somewhat different approach, a group at the California Institute of Technology (Caltech) developed a multimodal sensor that targets assessment of the stress levels of an individual. Their small, thin adhesive sensor, worn on the wrist, is dubbed CARES (consolidated artificial-intelligence-reinforced electronic skin). It allows users to participate in all normal daily activities with minimal interference during testing, making it possible to measure both baseline and acute levels of stress. It uses sweat to identify and measure physiological conditions (Fig. 6).
Utilizing microfluidic sampling on a wearable sweat sensor, this patch monitors three vital signs (pulse waveform, galvanic skin response, and skin temperature) and six molecular biomarkers in human sweat (glucose, lactate, uric acid, sodium ions, potassium ions, and ammonium). Machine-learning (ML) algorithms complete the stress analysis.
Previous materials used for sweat sensors could be produced efficiently using inkjet printing; they were capable of accurate measurement of even very scarce compounds. However, those materials gradually broke down in the presence of bodily fluids. The Caltech team added a nickel-based compound that helps stabilize the enzymatic-based sensors, like those that detect lactate or glucose, as does a new polymer added to the ion-based sensors, which detect biomarkers like sodium or potassium.
To develop the ML algorithms, they performed experiments to induce stress in subjects wearing the CARES device to demonstrate that the sensor accurately measures the interrelated-ness of physiological (such as pulse) and chemical (such as glucose) biomarkers. Subjects also answered questionnaires to self-report their feelings of anxiety and psychological stress before and after exposure to stressful situations like vigorous exercise or intense video gameplay.
Using these ML algorithms, the researchers maintain that the platform can differentiate among the stressors with an accuracy of 98.0% and quantify psychological stress responses with a statistical (if not actual) confidence level of 98.7%. You can read about their work in the paper “A physicochemical-sensing electronic skin for stress response monitoring,” published in Nature.