Members can download this article in PDF format.
What you'll learn:
- Advances in lithium-based technology.
- Battery chemistries that go beyond the lithium-ion boundary.
- New solid-state battery breakthroughs.
- Aluminum sulfur and molten salt?
In this article, we'll explore some of the most promising advanced battery technologies expected to come to market within the next few years, and how they plan to deliver dramatically lower cost per kilowatt-hour, higher power densities, and longer service lives. In the process, we’ll also look at whether their unique characteristics will require designers to take new approaches to how they’re charged, managed, and protected.
Breaking the Lithium Barrier
Lithium-ion (Li-ion) batteries have evolved rapidly since the pioneering work2 that eventually enabled Sony and Asahi Kasei to release the first commercial Li-ion battery in 1991. Since then, their performance has steadily improved while cost has declined, due to technical improvements and the economies of scale, made possible by a rapidly growing market. A recent Bloomberg-NEF (BNEF) analysis reported that the average cost of a Li-ion electric-vehicle (EV) battery pack dropped by 89% from $1,200/kWh in 2010 to $132/kWh in 2021.3
Despite their maturity, today's Li-ion architectures still suffer from several inherent problems that will eventually limit their power density and longevity. In addition, the lithium, cobalt, and other minerals used in their construction are currently subject to economic and geopolitical issues that will keep their cost relatively high and continue to cause supply-chain problems for the near-future. 4
The growing demand for batteries that overcome these issues has prompted the emergence of a surprising number of new technologies that can be roughly classified into a few broad categories:
- “Evolutionary” battery architectures that can be built using existing manufacturing infrastructures with more durable electrode structures, improved electrolytes, and other features supporting 5X-20X more charge-discharge cycles and significantly higher energy densities.
- “Revolutionary” batteries that reduce or eliminate the need for lithium and other costly or sensitive materials in existing high-capacity applications such as EVs.
- New architectures that use abundant, inexpensive materials to create long-life, low-cost, MWh-scale stationary batteries to supplement residential-, industrial- and grid-scale renewable-energy sources.
Building on Success
Lithium-based battery technologies dominate today's market for most applications, with nearly 225 GWh worth of capacity manufactured for EVs alone in 2021.4 Eventually, low-/no-lithium battery chemistries should lead to lower-cost products that exceed Li-ion's energy density. However, for the near-term, battery manufacturers are looking for incremental improvements to delay the need to write off the huge investments they've made in their existing factories.5
“This is the most likely outcome as the move to electric vehicles continues to accelerate and many of the world's major economies’ plans to move to renewable energy become realities,” said Dr. Yevgen Barsukov, Ph.D., Algorithm Fellow, Battery Management Systems at Texas Instruments.
He added, “Meeting the anticipated 20X growth in the demand for high-performance batteries in the next 10-15 years is a giant undertaking which will require the industry to focus on building the fabs, training the personal, and finding raw materials to support the existing supply chains. Some changes might be forced, such as moving from graphite to Si, or from Ni to Fe, but these are incremental improvements rather than going to completely new chemistry.”
To satisfy this demand, there has been a surge in research to develop and commercialize “transitional” materials and processes that can be quickly and (relatively) inexpensively integrated into existing Li-ion cells’ production lines. Some of these improvements involve new electrode materials, such as high-nickel cathodes and silicon and metallic lithium anodes, while others offer novel liquid, solid-state, and hybrid electrolyte formulations.
While by no means complete, these companies provide a representative sampling of the most promising approaches to taking the present generation of commercial Li-ion cells to the next level:
Addionics
Addionics has developed a chemistry-agnostic process for forming 3D electrode structures that it says improves a cell's capacity while offering dramatically improved internal resistance, heat dissipation, and internal mechanical stability. The company’s porous electrodes also enable higher energy densities because they can be saturated with more of the active battery material.
The 3D metal framework used to hold the anode chemistry, and its accompanying anode structure, greatly reduce the effects of the mechanical expansion/contraction that occur during charge/discharge cycles, as well as the formation of life-limiting dendrites. This enabled Addionics' first green prototype cell pouch (622 NMC and graphite) to retain 80% of its capacity after 2,000 charge/discharge cycles, as compared to a typical 2D foil cell to drop to roughly 60% capacity after 400 cycles.
The technology can be applied to most Li-ion chemistries (including lithium-iron-phosphate LFP), and most cylindrical and pouch form factors. At present, the company's focus is on products for EVs, with a partnership underway with three leading automotive OEMs to develop an NMC EV battery.
AM Batteries
A manufacturing technology for Li-ion batteries being developed by AM Batteries uses a lower-cost dry-electrode that also reduces their carbon footprint. The company’s process for coating Li-ion battery electrodes without the need for harmful solvents or energy-intensive evaporation is intended to solve the cost, scale, and environmental issues surrounding battery manufacturing, especially for EV OEMs.
Enovix
Enovix offers a unique electrode technology that also uses a 3D architecture. It’s based on a silicon-lithium alloy (Li15Si4) electrode material assembled as a micro-matrix that offers much higher energy density and requires much less lithium per kWh of capacity. The cell also incorporates features to prevent catastrophic failures by limiting the short-circuit currents that often lead to thermal runaway in the event the battery is punctured or crushed.
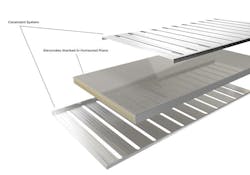
Furthermore, the Si/Li electrode structure greatly reduces the mechanical expansion most silicon-based electrodes undergo during charging that severely limits the number of charge-discharge cycles they can sustain. The electrodes are laser-patterned into 3- × 30-mm strips and stacked side-by-side orthogonally to the largest face of the battery.
The shorter stack length reduces the amount of force produced during swelling it undergoes during charging. Therefore, it can be easily managed by a stainless-steel constraint system that also improves the cell's durability. As a result, Evonix reports that the typical swelling of its 3D cell after 500 cycles is less than 2%.
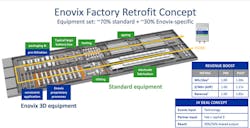
The company and its technology was originally introduced to Electronic Design readers in the November 16 edition of PowerBites5 when it was announced that the first battery cells have emerged from its automated factory in Fremont, Calif., on the path toward commercial production in 2022.
More importantly, the same cells can be produced in existing conventional Li-ion battery plants using roughly 70% of the already-installed manufacturing equipment. The remainder will be performed by Enovix’s proprietary roll-to-stack production tools, which are “drop-in” replacements for the winding or cut-and-stack tools used to make conventional pouch-style Li-ion cells.
NanoGraf
NanoGraf has developed advanced battery materials and an architecture that can use existing Li-ion production lines to build extremely energy-dense Li-ion cells in the same form factors as today's commercial products. The proprietary doped core composition and surface technology significantly improves stability and can be applied to the substrate without a complex (i.e., costly) vapor-deposition process.
The company's recently announced 18650 cell design, which uses its second generation of silicon-oxide anode material, demonstrated an energy density of 810 Wh/L (4.0-Ah capacity), while behaving exactly like a conventional cell in terms of voltage, charge/discharge characteristics, etc. NanoGraf says its technology allows cell producers to make "drop-in" upgrades to their Li-ion manufacturing facilities with few, if any, additional production cost. At the time of this writing, the company anticipates mass production in a U.S.-based facility to begin in December 2022, with wide-scale adoption during 2023.
Easy Design-In Accelerates Adoption
All of the companies who responded to our questions say that cells produced using their battery architecture behave much like an ordinary Li-ion cell. They also can use the same ICs and design practices presently employed to implement charging, management, and safety systems for the battery packs they will be used in.
This, along with the low cost to implement them, is why many industry authorities, such as Canada's Mining and Environment Research Centre,6 expect these “transitional” technologies to dominate the battery-chemistry landscape for the next decade.
Advanced Chemistries for the Long Haul
Whether it's copper networks, CMOS transistors, or Quonset huts, incumbent technologies have a habit of sticking around far longer than we'd expect. This may be true for today's Li-ion batteries as well. However, between their dependence on sensitive, potentially costly materials, and the inherent limits of their materials’ performance, cost, and durability, they will eventually face competition from upstart technologies with “revolutionary” architectures offering the 4X-10X improvements manufacturers need to justify “forklift upgrades” to their production lines.
Drexel's Lithium-Sulfur Cell
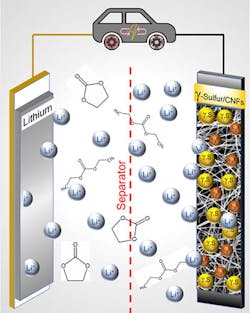
Drexel’s College of Engineering recently announced promising news about a sulfur-based battery architecture that may offer 3X the capacity of Li-ion batteries and last more than 4,000 recharges—far more than the 500-1,000 cycles typical of most Li-ion cells.7
Although its chemistry does include lithium, it requires a small fraction of the amount per kWh used by conventional batteries and eliminates the need for other scarce materials altogether. Their work, published in the journal Communications Chemistry,8 may offer a way to overcome those obstacles.
One of the problems with previous Li-S battery architectures is that they were subject to irreversible chemical reactions, resulting in nearly immediate shutdown and/or a complete failure of the battery, sometimes after a single charge/discharge cycle. Formulations using an ether-based electrolyte demonstrated exceptional performance in carefully controlled lab conditions, but any warming of the battery above room temperature could cause a failure or meltdown.
To solve this, Drexel's researchers developed a new way of producing and stabilizing a rare form of sulfur onto a carbon nanofiber matrix that serves as the battery's cathode. When used with a carbonate-based electrolyte, it forms the basis of a long-life battery architecture that remains stable across a wide temperature range.
After more than a year of testing, the sulfur cathode remains stable and, as the team reported, its performance has not degraded in 4,000 charge-discharge cycles—equivalent to 10 years of regular use. And, as predicted, the battery's capacity is more than threefold that of a Li-ion battery.
In a related development, a lithium-free sulfur-based cell that uses aluminum-based electrodes is under development at MIT, as reported in MIT News in August 2022.10
A High-Sodium Diet for a Healthy Planet?
A sodium-ion battery chemistry under development at the Department of Energy’s Pacific Northwest National Laboratory (PNNL)9 may provide the foundation for safe, rugged, and low-cost lithium-free batteries. Until now, sodium-ion batteries suffered from relatively short lives as due to its electrolyte causing irreversible erosion of the electrodes during discharge.
The findings, detailed in the journal Nature Energy, 11 explain how a novel salt-based electrolyte reacts to form a protective film on the battery's sodium-nitrogen-manganese-carbon (NaNMC) cathode. It allows sodium ions to pass through during charge and discharge while minimizing electrode corrosion.
The researchers admit that, for now, the sodium-ion technology still lags behind lithium in energy density. But it has its own advantages, such as imperviousness to temperature changes, stability, and long cycle life, which are valuable for applications of certain light-duty electric vehicles and even grid energy storage in the future.
Solid-State Solutions on the Horizon
“Conventional Li-ion batteries may be approaching a bottleneck, creating the opportunity for solid-state batteries (SSBs) which use solid-, or semi-solid, electrolytes to challenge incumbent technologies,” said Texas Instruments’ Dr. Yevgen Barsukov.
But it may take a while before SSBs are sufficiently mature to achieve their potential. Barsukov explains that while today's solid electrolytes using metallic lithium cathodes deliver acceptable performance, the brittle ceramic membranes they’re based on are difficult to manufacture in high-volume production quantities and suffer from other reliability issues.
He also notes that developers also must overcome a number of problems with the current generation of cathode and anode materials. Thus, it’s likely that the first generation of commercial SSBs will employ a hybrid structure that puts a solid electrolyte in contact with the anode, and a liquid or gel surrounding the cathode.
Barsukov expects that SSBs will overcome these challenges and find commercial applications within the next 5-10 years, although not necessarily as a low-cost option. Rather, they will find homes in areas where they’re absolutely needed, such as high-temperature environments or aviation.
But this timeline is only an estimate. We may see SSBs coming to market sooner if one of the many recent startup companies involved with SSB development, such as those noted here, achieves a breakthrough:
Adden Energy
Founded by a team of scientists at Harvard University, Adden Energy is developing and scaling up a solid-state battery that uses an advanced strain-stabilized ceramic-sulfide electrolyte,12 lithium-based electrodes, and a multi-electrolyte-layer separator. The latter enables rapid charging while inhibiting the formation of life-limiting dendrites that plague many solid-state architectures.
AI algorithms were employed to develop several other architectural features that can be used with high-current-density lithium metal anodes as well as high-voltage cathodes. A good overview of the tech was published by Medium13 earlier this year.
On its way to developing commercially viable amp-hour-sized cells, the company's lab-scale prototypes have already demonstrated charge times as low as three minutes, and capacity retention for over 10,000 cycles. Although we were unable to contact Adden for additional information, there are links to several scientific articles that provide important details about the architecture available on the company’s site (https://www.addenenergy.com/tech).
Theion
A solid-state lithium-sulfur battery architecture developed by Theion combines sulfur’s crystal material properties with carbon nanotubes and a proprietary solid electrolyte. The sulfur cathode material is manufactured using what the company calls a Direct Crystal Imprinting (DCi) method, which requires no slurry coating, solvents, water, or drying. As a result, a solid wafer can be formed in any shape or form factor directly from molten sulfur within a few seconds.
Theion said its batteries will offer low cost per kWh of storage, fast-charging characteristics, and up to triple the gravimetric and volumetric energy density of today's Li-ion cells. In addition, the manufacturing process is claimed to need as little as 10% of the energy required to produce an equivalent Li-ion cell.
With early samples anticipated to be delivered to alpha customers in late 2022, Theion says that its products will eventually be able to deliver 2,000 W/kg and maintain near-full capacity for 1,000+ charge cycles (at a 1C charge rate) across its −20 to 60°C operational temperature range. Electronic Design was unable to contact Theion for additional details at this time, but stay tuned for future developments.
What Designers Need to Know
Conversations with several industry experts indicate that these new battery architectures won't be drop-in replacements for the Li-ion cells currently used in EVs and other applications. It's too early to provide any details about the circuits that designers will need create to charge, manage, and protect these advanced batteries, but they were able to offer some useful insights about what we can expect.
TI's Barsukov said one of the issues to confront designers is that these new chemistries will exhibit very different charge/voltage profiles, possibly requiring significant changes to how they’re monitored and managed. For example, it's likely that many advanced battery chemistries will have an extremely flat discharge curve, even flatter than today's LFP cells. This will require even more accurate measurement techniques to monitor their state of charge.
Barsukov also notes that other issues may arise related to the lower voltages produced by some of these cells, often due to the novel compositions of their electrodes. For example, low-nickel or nickel-free electrodes produce closer to 2.5 V versus the roughly 3 V from typical NMC/graphite or LFP chemistries of other high-nickel electrodes.
This phenomenon may require the use of monitoring and balancing electronics that can function accurately within a lower operating range. Designers also should note that while stacking lower-voltage cells in series can be used to compensate for their lower voltage, such a configuration makes the use of buck-boost converters problematic.
Regardless of chemistry, the batteries used in tomorrow's vehicles and other applications will require dramatically faster charging rates, and the higher currents/voltage levels that accompany them. This will require smarter chargers that incorporate electronic devices, advanced algorithms, and design techniques to ensure these batteries don't experience premature aging and provide high levels of safety during high-power charge cycles.
For example, the most common CC/CV (constant current/constant voltage) charging algorithms are compromises between delivering the shortest possible charge rate and best longevity. Using a fixed-current charging scheme trades off a simpler design that has a longer (roughly 30%) charge time because it can’t take advantage of the higher current levels available at the beginning of the charge cycle.
On the other hand, a CC/CV charging scheme works well while the battery is new. However, as the cells age (often after only 20% capacity loss), their anode impedance increases and the same constant current can cause excessive Li-plating, thereby further accelerating its capacity loss during each subsequent charge cycle.
To avoid these issues, TI's Dr. Barsukov recommends using charging circuits that implement “smart” current control algorithms, which prevent Li-plating at all stages of a battery's life by monitoring the cells’ “health” and adjusting its charge-current profile accordingly. He notes that TI has already incorporated this capability into its MaxLife technology, found in a growing number of its gauge/charge products.
Regardless of chemistry, the batteries used in tomorrow's vehicles and other applications will require dramatically faster charging rates, and the higher currents/voltage levels that accompany them. "This requires the use of more sophisticated microprocessors and new protocols to communicate between the charger and the battery about what each side needs to do to safely bring the battery to its full charge state," said Gregory Green, Director of Automotive Customer Programs, Vicor Corp. "On a related topic, the industry is currently exploring several architectural design paths to provide accessory power to the car while it’s charging."
Green continued, "When you’re charging an EV for 30 minutes, you have to assume that, for some of that time, the driver might want to listen to the radio, use the Wi-Fi hotspot, or have the HVAC running. Not to mention–and most importantly–the battery needs to be cooled and monitored while it’s being recharged. Trying to shift power off the top while you’re pumping power in from the bottom is not a good answer, so we need to look at ways to have either lower-voltage batteries that make that transition or siphon off some charger power and route that to the vehicle's low-voltage power bus."
Go Big, Go with the Flow, or Go Dark
In addition to better, cheaper batteries for EVs and other mobile applications, a low-/no-carbon economy will require new storage technologies that can be used to smooth the variable outputs of grid-scale wind and solar farms. New battery technologies also will be used in renewable-equipped homes, offices, and industrial settings as part of smart, distributed grids.
Some of this capacity will be provided by fleets of containerized used EV batteries, which are usually “retired” when their capacity drops to 80%. As the global EV fleet matures, they will provide a steady stream of cells for these cost-effective storage modules, but it will still represent a fraction of the growing demand. The remainder will need to be satisfied with innovative battery technologies capable of producing cells with very large capacities (measured in MWh and GWh) that are both cost-effective and have service lives measured in decades.
The advances in industry-/utility-scale batteries represent a second storage revolution that will be covered in an upcoming article. Until then, we’ll close with a preview of some of the technologies we can look forward to coming on line in the surprisingly-near-future.
MIT's Aluminum-Sulfur Cell
A team of researchers from several universities have developed a lithium-free battery architecture that uses aluminum and sulfur as its two electrode materials, with a molten salt electrolyte in between. In theory, aluminum-sulfur chemistries can be used to make low-cost cells with high energy densities, rapid charging, and the ability to sustain thousands of charge/discharge cycles. They also can eliminate the need for volatile, flammable, and corrosive organic liquids, commonly used in most Li-ion batteries.
In reality, achieving a viable Li-S cell required the team to find salts that have the right electrochemical properties and relatively low melting points (e.g., close to the boiling point of water vs. nearly 1,000°F for many salts). MIT’s cell uses a chloro-aluminate salt that becomes molten at 25°C/77°F and achieves its peak performance at 110°C/230°F. Most of the heat required to keep the electrolyte in its molten state is expected to be produced by the battery itself while it is being charged and discharged.
Additional details of the Al-S battery architecture are described in paper by MIT Professor Donald Sadoway, along with 15 others at MIT and in China, Canada, Kentucky, and Tennessee, published in the journal Nature.14
Initial tests have shown the battery cells can endure hundreds of cycles at exceptionally high charging rates, with a projected cost per cell of about one-sixth that of comparable lithium-ion cells. In addition, the molten chloro-aluminate electrolyte also suppresses the formation of dendrites, narrow spikes of metal which build up and eventually create micro-short-circuits and that reduce a battery's capacity and efficiency.
Dr. Sadoway says that their low cost and low operating temperature makes them well-suited for storage systems with a few tens of kilowatt-hours of storage capacity, such as homes and small to medium business. They also will fit with technologies that have even higher energy densities, such the liquid-metal batteries being used for larger installations, of tens to hundreds of MWh.
PNNL's Freeze-Thaw Molten Salt Battery Captures Seasonal Sunshine
Low-cost salt-based batteries will probably be the leading technology for providing the MWh/GWh required by energy-storage utilities. However, they still suffer from self-discharge losses that make them impractical for holding surplus power in reserve for more than a few weeks at a time.
A new freeze-thaw molten battery, currently under development at the U.S. Department of Energy’s PNNL may eventually enable utilities to charge cells based on a hot liquefied salt electrolyte with excess power generated during peak sun and wind conditions, which revert to a solid at room temperature.15 In this state, the cell’s charge can be held with near-zero losses for months at a time for use whenever it’s most needed.
Guosheng Li, the project's principal investigator at PNNL, says that the technology is in the early stages of development, so there’s no clearly defined path to commercial development at this time. But the initial results, recently documented in Scientific American,16 are very promising and don’t reveal any insurmountable technical obstacles to its eventual use in tomorrow’s resilient power grids.
References
1. Lee Goldberg, Product Design & Development, Aug/Sept 2017: "Designing a Greener Bottom Line: Will our next wave of prosperity be driven by products and technologies that can halt climate change?" https://secureservercdn.net/50.62.89.104/17p.167.myftpupload.com/wp-content/uploads/2021/02/[email protected]
2. https://en.wikipedia.org/wiki/History_of_the_lithium-ion_battery
3. “China and Lithium Geopolitics in a Changing Global Market,” https://link.springer.com/article/10.1007/s41111-022-00227-3 Battery Chemistries for ElectricVehicles—Mini Review. Batteries 2022, 8, 70. https://doi.org/10.3390/batteries8070070
4. “Battery Pack Prices Fall to an Average of $132/kWh, But Rising Commodity Prices Start to Bite,” BloombergNEF. https://batteriesnews.com/battery-pack-prices-fall-132-kwh-commodity-prices-bloomberg/
6. Mohamed S. E. Houache et al, “On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review,” National Research Council of Canada, Energy, Mining and Environment Research Centre, July, 2022. https://www.mdpi.com/2313-0105/8/7/70
7. “Breakthrough in Cathode Chemistry Clears Path for Lithium-Sulfur Batteries’ Commercial Viability,” Drexel News, Feb 22, 2022. https://drexel.edu/news/archive/2022/february/lithium-sulfur-cathode-carbonate-electrolyte
8. Pai, R., Singh, A., Tang, M.H., et al. “Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li-S batteries,” Commun Chem 5, 17 (2022). https://doi.org/10.1038/s42004-022-00626-2
9. David Chandler, “A new concept for low-cost batteries,” MIT News, 8/24/2022. https://news.mit.edu/2022/aluminum-sulfur-battery-0824
10. Karyn Hede, “Longer Lasting Sodium-Ion Batteries on the Horizon,” PNNL News. https://www.pnnl.gov/news-media/longer-lasting-sodium-ion-batteries-horizon
11. Jin, Y., Le, P.M.L., Gao, P. et al. “Low-solvation electrolytes for high-voltage sodium-ion batteries,” Nat Energy 7, 718–725 (2022). https://doi.org/10.1038/s41560-022-01055-0
12. Wu, F., Fitzhugh, W., Ye, L. et al. “Advanced sulfide solid electrolyte by core-shell structural design,” Nat Commun 9, 4037 (2018). https://doi.org/10.1038/s41467-018-06123-2
13. Will Lockett, “Harvard’s Ingenious Groundbreaking Battery Will Shake-Up The EV World,” Medium, Sep. 2022. https://medium.com/predict/harvards-ingenious-groundbreaking-battery-will-shake-up-the-ev-world-84127236f048
14. Pang, Q., Meng, J., Gupta, S. et al. “Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting,” Nature 608, 704–711 (2022). https://doi.org/10.1038/s41586-022-04983-9
15. PNNL News, April 4, 2022, “Freeze-Thaw Battery Is Adept at Preserving Its Energy.” https://www.pnnl.gov › news-media › freeze-thaw-battery-adept-preserving-its-energy
16. Anna Blaustein, “Rechargeable Molten Salt Battery Freezes Energy in Place for Long-Term Storage,” Scientific American, May 6, 2022. https://www.scientificamerican.com/article/rechargeable-molten-salt-battery-freezes-energy-in-place-for-long-term-storage/